Pharmacovigilance in Crisis: Automation as the Answer to Workforce Shortages
HIT Consultant
MARCH 21, 2024
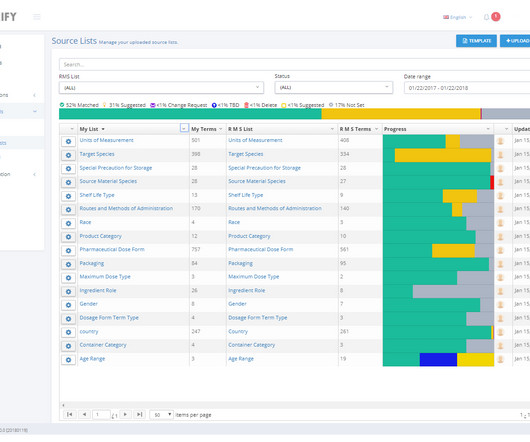
This loss of labor and expertise will significantly impact the pharmacovigilance (PV) space – especially as the field traditionally incorporates many manual processes to facilitate regulatory compliance and ensure patient safety. Without these industry experts, there are a plethora of consequences.












Let's personalize your content