Mary Madison, RN, RAC-CT, CDP
Clinical Consultant – Briggs Healthcare
With the date of March 10, 2022, CMS quietly updated QSO-20-38-NH.

[FYI NOTE: Last week, I reported that CMS had posted an updated Nursing Home Visitation FAQ document on March 10, 2022. Trying to locate a corresponding QSO, I found the revised QSO Memorandum further down in Policy & Memos to States and Regions website:

The September 2021 revision was in chronological order (the original QSO is August 26, 2020) as shown above however the March 10, 2022 revision is not obvious nor in chronological order – it’s beneath the September 2021 revision with no indication of the March update until you click on the 2nd hyperlink.]
There is a new definition of Up-to-Date for F886 found on page 3 of the 12-page QSO.
There’s revised information on pages 4 and 6:
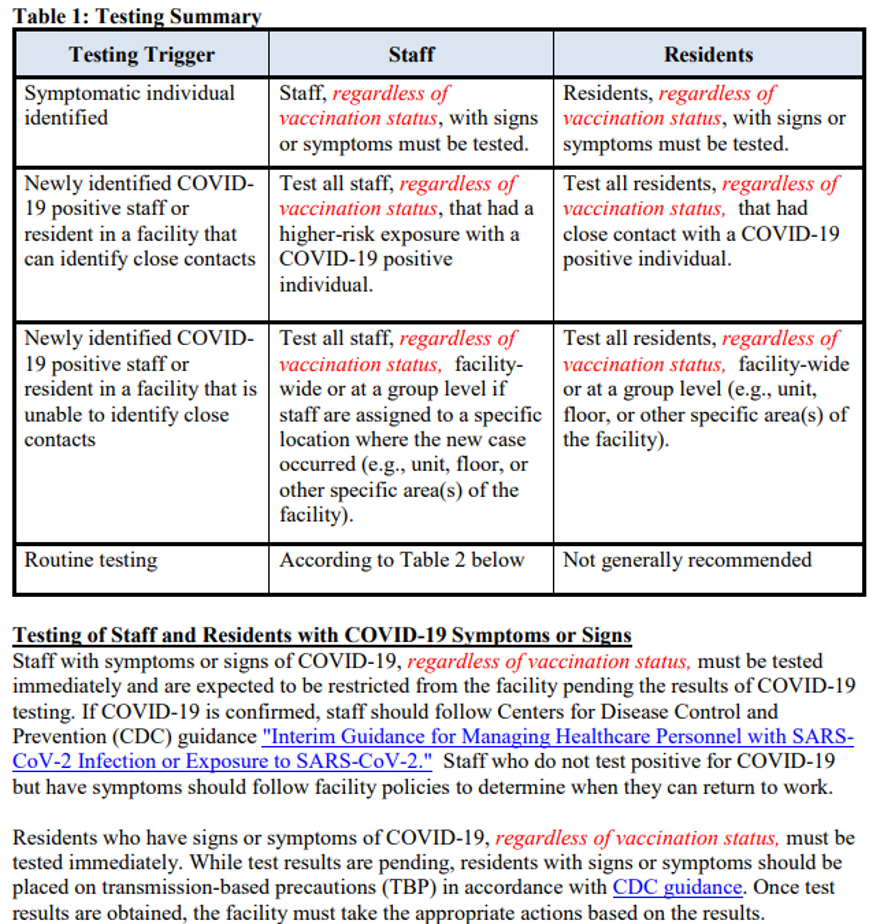

You’ll also find red ink on pages 7, 8 and 10. Below is what’s been updated on page 8:


One thought on “CMS Revises QSO-20-38-NH: LTC Facility COVID-19 Testing Requirements”
Comments are closed.