Mary Madison, RN, RAC-CT, CDP
Clinical Consultant – Briggs Healthcare
CMS posted QSO-22-04-ALL the afternoon of December 2, 2021.
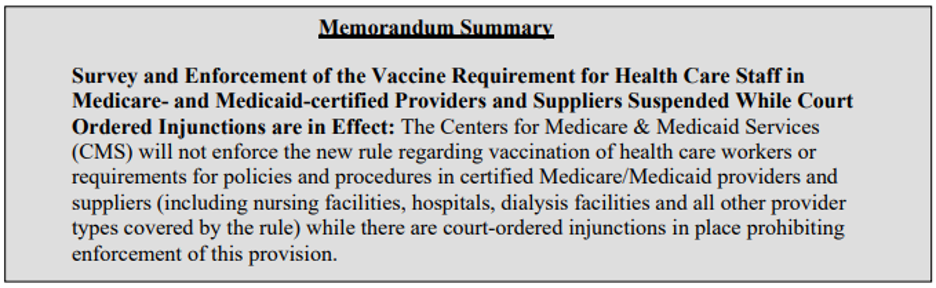
Here’s an excerpt from that QSO Memorandum (bolding and italics added by me): “On November 29 and November 30, 2021, the United States District Court for the Eastern District of Missouri and United States District Court for the Western District of Louisiana issued preliminary injunctions against the implementation and enforcement of the Interim Final Rule against Medicare and Medicaid-certified providers and suppliers. Between the two of them, these injunctions cover all states, the District of Columbia and the US Territories. CMS has appealed both of these decisions, and has filed motions for stays of these orders. While CMS remains confident in its authority to protect the health and safety of patients in facilities certified by the Medicare and Medicaid programs, it has suspended activities related to the implementation and enforcement of this rule pending future developments in the litigation. Accordingly, while these preliminary injunctions are in effect, surveyors must not survey providers for compliance with the requirements of the Interim Final Rule. Health care facilities, of course, may voluntarily choose to comply with the Interim Final Rule.

One thought on “Vaccination Regulation: Enforcement of Rule Imposing Vaccine Requirement for Health Care Staff in Medicare- and Medicaid-certified Providers and Suppliers is Suspended so Long as Court Ordered Injunctions Remain in Effect”
Comments are closed.